2013-11-11
org.kosen.entty.User@799587a4
류현모(kuki0127)
- 2
지금 isopropyl lactate 시약을 살려고 하니 마땅한 구매처가 없어서 lactic acid 를 에스터 반응시켜서 isopropyl lactate를 합성할려고 하는데 마땅한 자료가 없네요..
그리고 azeotropic reflux 상태에서 실험하라고 되어있는데 구체적으로 어떻게 실험을 하는거죠?
그리고 azeotropic reflux 상태에서 실험하라고 되어있는데 구체적으로 어떻게 실험을 하는거죠?
- azeotropic reflux
- isopropyl lactate
- lactic acid
지식의 출발은 질문, 모든 지식의 완성은 답변!
각 분야 한인연구자와 현업 전문가분들의 답변을 기다립니다.
각 분야 한인연구자와 현업 전문가분들의 답변을 기다립니다.
답변 2
-
답변
조윤환님의 답변
2013-11-11- 0
반갑습니다.
추측컨데 lactic acid와 isopropyl alcohol(IPA)을 산촉매 존재하에서 반응시키는 Fischer esterification protocol을 참고하신게 아닌가 싶습니다.
반응의 특성상 반응이 진행되면 물이 생성되고 물로 인해서 역반응이 일어날 수 있기 때문에 생성되는 물을 제거해 주어야 효율을 높일 수 있습니다. 보통 산촉매로 황산이나 인산을 사용하죠.
생성되는 물을 제거하기 위해 사용하는 방법이 바로 azotropic distillation입니다. 반응 혼합물에 benzene이나 toluene 같은 용매를 넣고 reflux를 하면 물+IPA+benzene 과 같은 3성분 azotrope가 형성되서 낮은 온도에서 함께 끓어 오릅니다. 이와 같은 원리로 생성된 물이 반응시스템에서 쉽게 제거되는 것입니다.
따라서 그냥 reflux를 하시면 않되고 증류를 해내어야 합니다. reflux는 응축된 용매가 다시 반응기 안으로 되돌아가기 때문입니다.
Benzene은 독성 때문에 사용을 기피하는 용매이므로 toluene이나 cyclohexane을 사용하시면 되겠습니다. IPA 때문에 어떻게 될지 확언할 수는 없지만 응축된 azotrope에서 물과 유기용매 상이 분리가 일어나는 경우라면 Dean-Stark trap을 이용하면 증류를 해내지 않고 환류시킬 수도 있으니 참고하시기 바랍니다. -
답변
이배훈님의 답변
2013-11-11- 0
Organic Syntheses. 1930, 10, 88ISOPROPYL LACTATE[Lactic acid, isopropyl ester]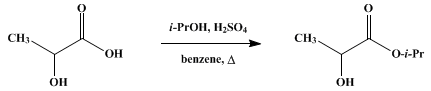 1. ProcedureIn a 3-l. round-bottomed flask (Note 1) fitted with a 1-meter fractionating column1 are placed 450 g. (7.5 moles) of anhydrous isopropyl alcohol (Note 2), 212 g. (2 moles) of u.s.p. 85 per cent lactic acid, 1 l. of benzene, and 5 cc. of concentrated sulfuric acid. The flask is placed in an air bath on an electric hot plate (Note 3) and heated until the benzene-isopropyl alcohol-water ternary mixture distils at 66.5°. Distillation is continued slowly (six to seven hours) until the temperature at the head of the column rises to and persists at 71–72° (isopropyl alcohol-benzene binary mixture), and no further separation of water occurs. Ten grams of precipitated calcium carbonate is then added to the mixture, and distillation is continued until the temperature rises to 80° in order to remove most of the benzene and excess isopropyl alcohol (Note 4) and (Note 5). The contents of the flask are then filtered into a modified Claisen flaskand distilled under reduced pressure. Cuts are taken to 60°, 60–75°, 75–80°, and 80–100° at 32 mm. The fraction boiling at 75–80°/32 mm. is isopropyl lactate and weighs 130–160 g. By redistilling the high and low fractions an additional 30–60 g. is obtained, bringing the total yield to 160–180 g. (60–68 per cent of the theoretical amount). The ester may be redistilled at atmospheric pressure (with some loss due to decomposition) at 166–168°.2. Notes1. With larger amounts of material it is desirable to employ a two-necked flask; the spare neck is used for introducing the calcium carbonate later in the process.2. Commercial anhydrous alcohol was used in this preparation. Isopropyl alcohol is very difficult to dry satisfactorily. The water binary mixture, boiling at 80.35°, contains 12.1 per cent of water by weight. The ternary mixture with benzene, boiling at 66.5°, contains 73.8 per cent benzene, 18.7 per cent isopropyl alcohol, and 7.5 per cent water. Hence by adding 120 g. of dry benzene to 100 g. of the isopropyl alcohol-water binary mixture, and distilling until the temperature reaches 82°, there will remain 55 to 60 g. of nearly dry isopropyl alcohol.3. A water or steam bath or oil bath may be used.4. The temperature of the vapors should not be allowed to rise above 72° before the addition of the calcium carbonate. If too much alcohol is removed before the acid is neutralized, charring and resinification take place with a decrease in the yield of ester.5. The recovered benzene and excess isopropyl alcohol may be dried by distillation and used in a subsequent run.3. DiscussionIsopropyl lactate has been prepared by heating isopropyl alcohol and lactic acid in a sealed tube at 170°,2 and from silver lactate and isopropyl iodide, together with the isopropyl ester of α-isopropoxypropionic acid.3 Direct esterification of lactic acid with isopropyl alcohol, using sulfuric acid, has hitherto given less than a 20 per cent yield of impure ester.References and Notes
1. ProcedureIn a 3-l. round-bottomed flask (Note 1) fitted with a 1-meter fractionating column1 are placed 450 g. (7.5 moles) of anhydrous isopropyl alcohol (Note 2), 212 g. (2 moles) of u.s.p. 85 per cent lactic acid, 1 l. of benzene, and 5 cc. of concentrated sulfuric acid. The flask is placed in an air bath on an electric hot plate (Note 3) and heated until the benzene-isopropyl alcohol-water ternary mixture distils at 66.5°. Distillation is continued slowly (six to seven hours) until the temperature at the head of the column rises to and persists at 71–72° (isopropyl alcohol-benzene binary mixture), and no further separation of water occurs. Ten grams of precipitated calcium carbonate is then added to the mixture, and distillation is continued until the temperature rises to 80° in order to remove most of the benzene and excess isopropyl alcohol (Note 4) and (Note 5). The contents of the flask are then filtered into a modified Claisen flaskand distilled under reduced pressure. Cuts are taken to 60°, 60–75°, 75–80°, and 80–100° at 32 mm. The fraction boiling at 75–80°/32 mm. is isopropyl lactate and weighs 130–160 g. By redistilling the high and low fractions an additional 30–60 g. is obtained, bringing the total yield to 160–180 g. (60–68 per cent of the theoretical amount). The ester may be redistilled at atmospheric pressure (with some loss due to decomposition) at 166–168°.2. Notes1. With larger amounts of material it is desirable to employ a two-necked flask; the spare neck is used for introducing the calcium carbonate later in the process.2. Commercial anhydrous alcohol was used in this preparation. Isopropyl alcohol is very difficult to dry satisfactorily. The water binary mixture, boiling at 80.35°, contains 12.1 per cent of water by weight. The ternary mixture with benzene, boiling at 66.5°, contains 73.8 per cent benzene, 18.7 per cent isopropyl alcohol, and 7.5 per cent water. Hence by adding 120 g. of dry benzene to 100 g. of the isopropyl alcohol-water binary mixture, and distilling until the temperature reaches 82°, there will remain 55 to 60 g. of nearly dry isopropyl alcohol.3. A water or steam bath or oil bath may be used.4. The temperature of the vapors should not be allowed to rise above 72° before the addition of the calcium carbonate. If too much alcohol is removed before the acid is neutralized, charring and resinification take place with a decrease in the yield of ester.5. The recovered benzene and excess isopropyl alcohol may be dried by distillation and used in a subsequent run.3. DiscussionIsopropyl lactate has been prepared by heating isopropyl alcohol and lactic acid in a sealed tube at 170°,2 and from silver lactate and isopropyl iodide, together with the isopropyl ester of α-isopropoxypropionic acid.3 Direct esterification of lactic acid with isopropyl alcohol, using sulfuric acid, has hitherto given less than a 20 per cent yield of impure ester.References and Notes- Clarke and Rahrs, Ind. Eng. Chem. 15, 349 (1923).
- Silva, Bull. soc. chim. (2) 17, 97 (1872).
- Purdie and Lander, J. Chem. Soc. 73, 298 (1898).
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)sulfuric acid (7664-93-9)
Benzene (71-43-2)
calcium carbonate (471-34-1)
isopropyl alcohol (67-63-0)
lactic acid (50-21-5)
Isopropyl lactate,
Lactic acid, isopropyl ester (63697-00-7)silver lactate (128-00-7)
isopropyl iodide (75-30-9)
isopropyl ester of α-isopropoxypropionic acid
Copyright © 1921-2013, Organic Syntheses, Inc. All Rights Reserved


