Inorganic-Organic Hybrid Perovskite Solar Cell
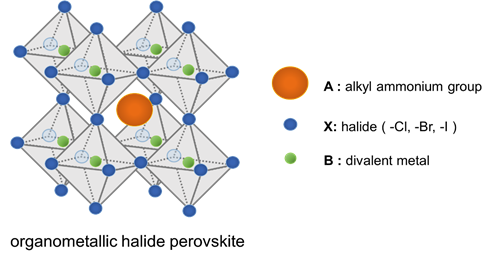
Organometallic halide Perovskite (ABX3) has alkyl ammonium group, divalent metal, halide at A, B and X, respectively. In these compounds, metal cations are in a 6-fold coordination and are surrounded by octahedron of halide anions together with the organic cations in a 12-fold cuboctahedral coordination. The perovskite materials have unique properties as good light-harvester of sensitized solar cell such as
- high absorption coefficient by direct bandgap
- small exciton binding energy (Wannier-type) due to high dielectric constant
- good charge collection by ambipolar characteristics
- long diffusion length of charge carriers by long time
- high open circuit voltage by small exciton binding energy.
- convenient bandgap tenability by controlling construction
- no formation of deep traps
- low temperature solution processibility.
If these points are applied to absorber of sensitized solar cell, hybrid perovskite solar cell can exhibit very high power conversion efficiency of over 20% at 1 sun condition.
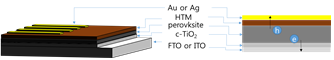
When device absorbed the light (photon) through glass, excitons are separated by holes and electrons in perovskite layer. Generated charge carriers (holes and electrons) move to each suitable layers such as hole transporting layer , electron transporting layer, and then charge carriers are accumulated. It works due to difference of each layer’s energy level, so we carefully choose materials for device fabrication. Finally, solar energy converts to electric energy after external circuit connected.
Recently, organic-inorganic hybrid perovskite solar cells undergo the spotlight and are actively studied in the whole world because of several advantages. For all that, hybrid perovskite solar cells aren’t commercialized yet, because some issues need to be solved.
- decomposed by humidity
- photostability problem
- J-V hysteresis with respect to the scan rate and direction
- toxicity of lead
After such issues are solved, we expect to use solar energy source instead of fossil fuels.

Organometallic halide Perovskite (ABX3) has alkyl ammonium group, divalent metal, halide at A, B and X, respectively. In these compounds, metal cations are in a 6-fold coordination and are surrounded by octahedron of halide anions together with the organic cations in a 12-fold cuboctahedral coordination. The perovskite materials have unique properties as good light-harvester of sensitized solar cell such as
- high absorption coefficient by direct bandgap
- small exciton binding energy (Wannier-type) due to high dielectric constant
- good charge collection by ambipolar characteristics
- long diffusion length of charge carriers by long time
- high open circuit voltage by small exciton binding energy.
- convenient bandgap tenability by controlling construction
- no formation of deep traps
- low temperature solution processibility.
If these points are applied to absorber of sensitized solar cell, hybrid perovskite solar cell can exhibit very high power conversion efficiency of over 20% at 1 sun condition.


When device absorbed the light (photon) through glass, excitons are separated by holes and electrons in perovskite layer. Generated charge carriers (holes and electrons) move to each suitable layers such as hole transporting layer , electron transporting layer, and then charge carriers are accumulated. It works due to difference of each layer’s energy level, so we carefully choose materials for device fabrication. Finally, solar energy converts to electric energy after external circuit connected.
Recently, organic-inorganic hybrid perovskite solar cells undergo the spotlight and are actively studied in the whole world because of several advantages. For all that, hybrid perovskite solar cells aren’t commercialized yet, because some issues need to be solved.
- decomposed by humidity
- photostability problem
- J-V hysteresis with respect to the scan rate and direction
- toxicity of lead
After such issues are solved, we expect to use solar energy source instead of fossil fuels.
Quantum dot (QD) sensitized solar cell
<structure cell="" dot="" of="" quantum="" sensitized="" solar=""></structure>
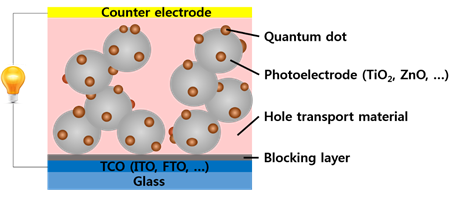
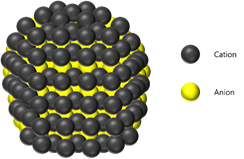
Quantum dot (QD) sensitized solar cell that composed with sensitizer (QD) to produce electrons and holes, photoelectrode and hole transport material for transmitting electrons and holes is composed of three core materials.
Quantum dot is prominent material due to convenient bandgap tunability, high absorption coefficient (105 M-1cm-1), multiple exciton generation, high dipole moment and low cost (solution process).
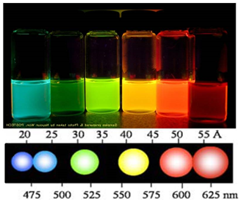
<quantum dot=""><<light different="" of="">wavelengths emitted by the QD size adjustment</light></quantum>>
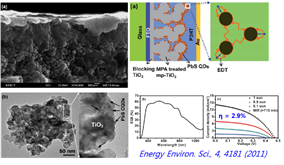
<pbs tio=""></pbs>
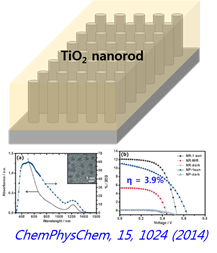
If we fabricate the QD solar cell based on the properties, we can get expected data that can absorb infrared region. And by changing QDs material properties and the structure, we can get higher conversion efficiency.
Recently, we remove a surface carbon chain ligand that impede transport of electrons and holes. And we use Successive Ionic Layer Adsorption and Reaction (SILAR) method, so QD can be deposited directly on the photoelectrode.
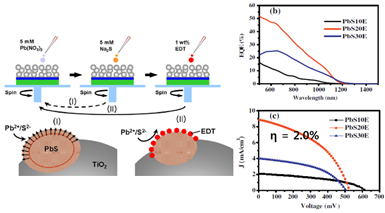
<pbs by="" deposited="" instead="" ligands="" method="" of="" qd="" silar="" surface="" synthesis="" using=""></pbs><pbs by="" deposited="" instead="" ligands="" method="" of="" qd="" silar="" surface="" synthesis="" using=""><pbs by="" deposited="" instead="" ligands="" method="" of="" qd="" silar="" surface="" synthesis="" using=""></pbs></pbs>

<structure cell="" dot="" of="" quantum="" sensitized="" solar=""></structure>
Quantum dot (QD) sensitized solar cell that composed with sensitizer (QD) to produce electrons and holes, photoelectrode and hole transport material for transmitting electrons and holes is composed of three core materials.
Quantum dot is prominent material due to convenient bandgap tunability, high absorption coefficient (105 M-1cm-1), multiple exciton generation, high dipole moment and low cost (solution process).


<quantum dot=""><<light different="" of="">wavelengths emitted by the QD size adjustment</light></quantum>>


<pbs tio=""></pbs>
If we fabricate the QD solar cell based on the properties, we can get expected data that can absorb infrared region. And by changing QDs material properties and the structure, we can get higher conversion efficiency.
Recently, we remove a surface carbon chain ligand that impede transport of electrons and holes. And we use Successive Ionic Layer Adsorption and Reaction (SILAR) method, so QD can be deposited directly on the photoelectrode.

<pbs by="" deposited="" instead="" ligands="" method="" of="" qd="" silar="" surface="" synthesis="" using=""></pbs><pbs by="" deposited="" instead="" ligands="" method="" of="" qd="" silar="" surface="" synthesis="" using=""><pbs by="" deposited="" instead="" ligands="" method="" of="" qd="" silar="" surface="" synthesis="" using=""></pbs></pbs>
Sb2S3– Sensitized Inorganic-Organic Heterojunction Solar cells
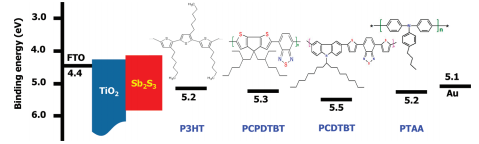
Among metal chalcogenides, Sb2S3 is appealing as an efficient light absorber because of its suitable band gap (≒ 1.7eV) and strong absorption coefficient ( 1.8×105
1.7eV) and strong absorption coefficient ( 1.8×105 in visible region). The representative method for the deposition of Sb2S3 light absorber is successive ionic layer adsorption and reaction (SILAR), chemical bath deposition (CBD), and spin-coating deposition.
in visible region). The representative method for the deposition of Sb2S3 light absorber is successive ionic layer adsorption and reaction (SILAR), chemical bath deposition (CBD), and spin-coating deposition.
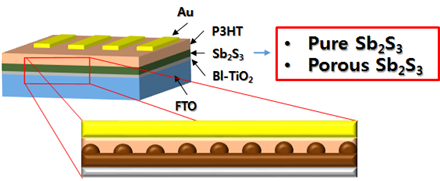
Although the SILAR and CBD in aqueous phase can form conformal thin film on a photoanode, it is difficult to avoid the formation of antimony oxides due to the formation of insoluble SbOCl so that the Sb2S3 light absorber reveals deep traps within bandgap. Therefore, additional healing process is required to convert the antimony oxide to antimony sulfide to eliminate/reduce the trap sites. So We add sulfur rich materials like Sb(TA)2Cl3 single source precursor(SSP), CuS or CuEDA after CBD process.
By using SSP, We can heal of the impure Sb2S3 light absorber and get precise thickness / morphology control. And by using Cu source , We can expect more efficiency because of the character of cu, acting like P type semiconductor.

Among metal chalcogenides, Sb2S3 is appealing as an efficient light absorber because of its suitable band gap (≒
 1.7eV) and strong absorption coefficient ( 1.8×105
1.7eV) and strong absorption coefficient ( 1.8×105 in visible region). The representative method for the deposition of Sb2S3 light absorber is successive ionic layer adsorption and reaction (SILAR), chemical bath deposition (CBD), and spin-coating deposition.
in visible region). The representative method for the deposition of Sb2S3 light absorber is successive ionic layer adsorption and reaction (SILAR), chemical bath deposition (CBD), and spin-coating deposition.
Although the SILAR and CBD in aqueous phase can form conformal thin film on a photoanode, it is difficult to avoid the formation of antimony oxides due to the formation of insoluble SbOCl so that the Sb2S3 light absorber reveals deep traps within bandgap. Therefore, additional healing process is required to convert the antimony oxide to antimony sulfide to eliminate/reduce the trap sites. So We add sulfur rich materials like Sb(TA)2Cl3 single source precursor(SSP), CuS or CuEDA after CBD process.
By using SSP, We can heal of the impure Sb2S3 light absorber and get precise thickness / morphology control. And by using Cu source , We can expect more efficiency because of the character of cu, acting like P type semiconductor.
국가
대한민국
소속기관
고려대학교 (학교)
연락처
02-3290-3295 http://necs.korea.ac.kr/main.php
책임자
임상혁 imromy@korea.aclkr



